SECOND INVESTMENT CYCLE
Details
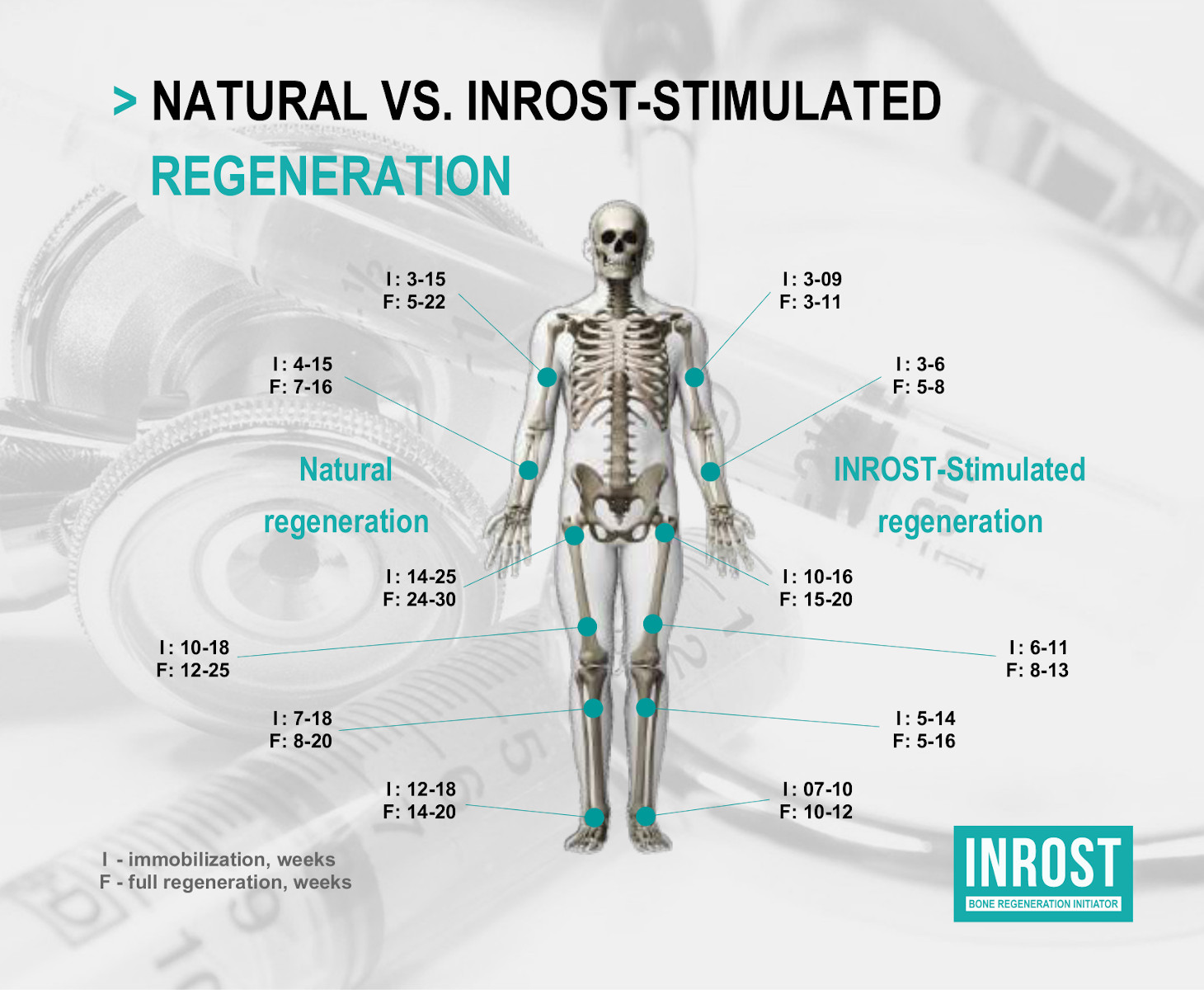
INROST is a therapeutic injection drug for bone regeneration. The drug significantly, up to two times, accelerates bone regeneration compared to other methods. As a result of the first investment cycle, initial preclinical trials and some additional studies have been completed in Russia, the effectiveness of the drug has been confirmed.
Additional studies have shown that the drug is not toxic, does not accumulate in the body, does not disrupt the natural physiological stages of healing and is genetically safe.

Patents have been obtained in the USA, Canada, China, United Kingdom, France, Germany, Japan and Russia. There are plans to obtain a patent in India.
Market Opportunity
Areas of application of the INROST: traumatology and orthopedics; plastic surgery; military medicine; sports traumatology; elite veterinary medicine.
The global available market for bone regeneration products is estimated at US$ 18 billion per annum, including US market reaching up to US$ 2 billion per annum.
The market for bone regeneration products has been growing in recent years, driven by an increasing demand for treatments for bone fractures, spinal fusion, and other bone-related injuries and conditions.
As the population ages, the incidence of conditions such as osteoporosis and osteoarthritis is also increasing, further driving demand for bone regeneration products.
MULTIPLIER EFFECT
The target funding sought for the Second Investment Cycle is US$ 13 million. The Second Investment Cycle will focus on preclinical studies in the US and obtaining FDA approval for clinical trials. According to the preparatory bidding process currently underway, preclinical studies will take 9 to 12 months depending on the protocols selected for the study in consultation with all the key stakeholders. The team, however reserved extra time to account for any justified delays that may occur.

Completion of the preclinical studies will increase the company's capitalization to US$ 300 million and significantly increase its investment appeal. Completion of clinical trials (which is beyond the scope of the Second Investment Cycle) will, in turn, increase the company's capitalization to US$ 1 billion.